Werner Heisenberg was the winner of the 1932 Nobel Prize in Physics and the creator of the Heisenberg Uncertainty Principle in Quantum Mechanics. Here is a recording of an interview he gave in the 1970's at the Max Planck Institute in Munich, Germany, describing how the nature of quantum mechanics goes beyond the limit of descriptive language and challenges our common sense notions of understanding.
While a student of Amold Sommerfeld at Munich in the early 1920s Werner Heisenberg (1901-76) first met the Danish physicist Niels Bohr. He and Bohr went for long hikes in the mountains and discussed the failure of existing theories to account for the new experimental results on the quantum structure of matter. Following these discussions Heisenberg plunged into several months of intensive theoretical research but met with continual frustration. Finally, suffering from a severe attack of hay fever, he retreated to the treeless island of Helgoland. After days spent relaxing and swimming Heisenberg suddenly experienced the giddy sensation of looking down into the heart of nature and conceived the basis of the quantum theory. He took this theory to Bohr at Copenhagen, and for the next few weeks they argued and probed its implications long into the night. The results of these discussions became known as the 'Copenhagen interpretation of quantum theory' and are accepted by most physicists. Aspects of the interpretation include Heisenberg's uncertainty principle and Bohr's principle of complementarity.
Heisenberg is best known for formulating the uncertainty principle, a fundamental contribution to the development of quantum theory. This principle states that it is impossible to accurately measure simultaneously the position and momentum of a particle.
In classical physics, we believe that we have full certainty of a system if we know the position and momentum of all particles at a given instant. When analyzing a system that consists of a single electron, Heisenberg imagined a theoretical configuration similar to the diagram on the left. This apparatus would be a gamma ray microscope that would try to determine precisely the position of the electron using high frequency photons. These high frequency photons would in turn interact with the electron to significantly alter its momentum. To try to determine the exact momentum we would then use lower energy photons, but this would alter minimally particle velocity hence giving us an overly "blurred" position.
In short, he found that there was no possible compromise that would allow us to accurately measure both variables. Generally, when a system is small enough, there is no physically possible to observe methods without significantly altering its state. The reason, returning to the example above, is because the photons used in the measurement are always quantised as photons and for a photon incident on a particle must have a peak wavelength corresponding to the diameter of the particle (otherwise the particle is transparent to photon) to interact. We know that the energy of a photon is inversely proportional to its wavelength, namely:
E = hc/λ quantifies the maximum accuracy we can expect to get from an observation: the total error in our measurements simultaneous two conjugate variables will always at least equal to Planck's constant divided by a factor of 4 times pi (3.141592654). Planck's constant corresponds to the quantum of action, that is, the minimum action that can have on a system. Hence even with the most sensitive equipment, the laws of physics have a minimal amount of uncertainty given in units of Planck's constant. This is the origin of Heisenberg's Uncertainty Principle.
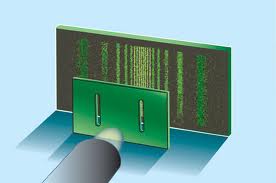
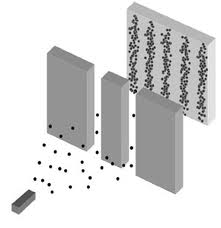
Another way to think about this is to also consider the famous double slit experiment. In this experiment, electrons, particles, enter a series of 2 slits and create an interference pattern just as if they are a wave. Even if the electrons are shot through one at a time, the path the electron traces out will cause it to interfere with itself.
One way to interpret this view is that the double slitted sheet acts as a particle detector. Unlike a light wave, the electron wave is not a wave of electric and magnetic forces but is a probability wave. The particle, as a wave, has known momentum, propagating the wave packet paralell to the detector. However when it enters one or other of the slits its position becomes known. Hence we see the momentum vector of the wave become spread out over a continuum of directions. Therefore, we might think the uncertainty principle can be seen as a consequence of the wave view of particles alone, and can be derived from the familiar wave mechanics of light.
The Double-Slit Experiment.
In the wave interpretation of particles, as the parallel probability wave of the electron propagates toward the detector we know its momentum (i.e. the Euclidean norm of the propagation vector) however its position is undefined in an infinity of possible positions along the wave peaks. When it enters either one of the slits its position is then known however the single momentum vector becomes blurred in an infinite number of projections and the wave then spreads out creating an interference pattern.
However ,there is more to the nature of quantum particles than they behave like waves some times but not others; as the great physicist Richard Feynman aptly put it "They behave in their own inimitable way, which technically could be called a quantum mechanical way. They behave in a way that is like nothing that you have seen before."
The path quantum particles takes is not always a straight line. Classical objects follow trajectories parallel to their momentum due to Hamilton's Principle of Least Action.
However, in quantum mechanics the Hamiltonian is quantised in units of Planck's constant. Once the position of the particle begins to reach the order of Planck's constant it will follow trajectories which deviate from the least action, i.e. a straight line trajectory. This explains more fully why particles appear to behave this way on the quantum scale rather than the macroscopic scale; there is no magical barrier to cross the classical to the quantum world, Planck's constant is small therefore quantum mechanics only behaves on small scales in space, time, energy and momentum.
(For a more detailed explanation of this see the notes section below and/or read Chapter 2 of Richard Feynman’s book, QED: The Strange Theory of Light and Matter, which uses this view to describe the behavior of photons)
To understand the uncertainty principle itself it is essential to reflect on the processes we call "observation" , or "measurement". When we make a measurement in an experiment, what we do is to try to extract information from a system by entering a meter that, by contact the observed system is altered by it. With the Uncertainty Principle it is clear that any act of measurement of a quantised system affects the system.
The uncertainty principle tells us that we can not simultaneously measure with infinite precision and a pair of conjugate variables. is, nothing prevents us to measure with infinite precision the position of a particle, but in doing so we have infinite uncertainty of its momentum over all possible time.
At that time Heisenberg first proposed this, there was general discussion among physicists about the possible ways to establish a coherent quantum theory, a coherent quantum mechanics. Among the many attempts, the most interesting for Heisenberg was the attempt of Hendrik Kramers to study the dispersion of atoms and, by doing so, to get some information about the amplitudes for the radiation of atoms.
In this connection, it occurred to Heisenberg that in the mathematical scheme these amplitudes behaved like the elements of a mathematical quantity called a matrix. So, working with Kramers, they both tried to apply a mathematical calculus to the light dispersion experiments. The result of which was the expression for finding the cross-section for a photon scattered of an atomic electron.
The quantum mechanical derivation was given by Paul Dirac in 1927.
The scary formula above represents the probability of the emission of photons of energy
 in the solid angle
in the solid angle  (centred in the
(centred in the  direction), after the excitation of the system with photons of energy
direction), after the excitation of the system with photons of energy  .
.  are the initial, intermediate and final states of the system with energy
are the initial, intermediate and final states of the system with energy 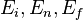 respectively; the delta function ensures the energy conservation during the whole process.
respectively; the delta function ensures the energy conservation during the whole process.  is the relevant transition operator.
is the relevant transition operator.  is the instrinsic linewidth of the intermediate state.
is the instrinsic linewidth of the intermediate state.The Kramers–Heisenberg formula was an important achievement when it was published, explaining the notion of stimulated emission, the necessary concept in the theory of lasers, and inelastic scattering - where the energy of the scattered photon may be larger or smaller than that of the incident photon - thereby anticipating the Raman effect.
From this it was clear that the first general quantum mechanical models describing the atom, turned out to be matrix mechanics.
Although matrix mechanics agreed with experiments it was not a popular theory. Matrices may now be part of the mathematics that are taught to physics and chemistry students at universities in their first year, but in the 1920's few non-mathematicians had heard of them. Moreover they had an odd property. If two physical properties, say A and B, are multiplied with each other, one would expect AB=BA. If these properties are replaced by matrices, then this suddenly is not necessarily true.
Around that time, the first papers of Erwin Schrödinger became known. Schrödinger tried to develop the idea of wave-particle duality, first proposed by Louis de Broglie, into a new mathematical scheme, which was called wave mechanics.
The famous Schrödinger Equation was actually able to treat the hydrogen atom on the basis of this wave mechanical scheme. This model was much more readily accepted then matrix mechanics, in part because the wave function could be visualized, and the theory was based on well-established classical mechanics.
The two conflicting notions of quantum mechanics, the matrix form of Heisenberg versus the wave form of Schrödinger meant that there was much disagreement between the two of them.
In fact the proponents of the matrix interpretation, Werner Heisenberg, Max Born and Niels Bohr blamed many widespread misconceptions about quantum mechanics on the popularity of the wave mechanics with little predictive power behind it.
Today, we usually use the terms "Schrödinger picture" and "Heisenberg picture" of quantum mechanics for the historical concepts wave mechanics and matrix mechanics, respectively.
Matrix mechanics and wave mechanics predict exactly the same results for experiments. This suggests that they are really different forms of a more general theory. In 1930 Paul Dirac showed that this is indeed the case. He gave a more general formulation of quantum mechanics; the one that is still used today in the form of Dirac's bra-ket notation for the wave function.consisting of a left part, 〈φ|, called the bra, and a right part, |ψ〉, called the ket.
The overlap expression 〈φ|ψ〉is typically interpreted as the probability amplitude for the state of the wavefunction ψ to collapse into the state ϕ.
Since bras and kets can be treated as row and column vectors respectively then it is understood that a bra next to a ket implies matrix multiplication. Hence the wave interpretation and matrix interpretation are equivalent and all of quantum physics follows from this.
In Dirac notation, the Schrödinger equation of time-evolution would be written as
Heisenberg also developed the first models describing the role of electron spin in magnetization dynamics. The Heisenberg model for magnetism is an extension of the Ising model and is a statistical mechanical model used in the study of critical points and phase transitions of magnetic systems, in which the spins of the electrons in magnetic systems are treated quantum mechanically.
The Heisenberg Model for magnetism, is given by the Heisenberg Hamiltonian:

where J is the exchange energy, and S represents a quantum spin operator. The summation is over nearest-neighbor spins; If J is positive, the spins Si and Sj tend to align ferromagnetically, If J is negative, the spins tend to allign anti-ferromagnetically.
This would be later extended into the Majumdar-Ghosh model and the Landau-Lifshitz model describing the quantum origins of magnetism in situations where the electron wavefunctions interact with each other in 1D, 2D and 3D.
Historically, when the Nazi government came to power in Germany, there was an innate distrust of what was vaguely called "Jewish Physics", some of which was the theoretical physics at the basis of quantum mechanics and relativity, due to the connections with Einstein and other famous Jewish physcisits. Some of Heisenberg's work came under scrutiny for this reason and vague though it was the supporters of the equally vague termed "Aryan Physics" attacked Heisenberg. One attack was published in Das Schwarze Korps, the newspaper of the Schutzstaffel (SS), headed by Heinrich Himmler. In this, Heisenberg was called a "White Jew" who should be made to "disappear". These attacks were taken seriously, as Jews and many other groups of society were violently attacked, discriminated and often incarcerated even before the height of the Nazi reign of terror which came to its climax with the Holocaust which would have been known to many of the higher echelons in Nazi dominated society. Heisenberg had to fight back with an editorial and a letter to Himmler, in an attempt to resolve this matter and save himself from persecution and possible imprisonment.
Controversially of course, Heisenberg was in charge of the scientific research project of the German atomic bomb during World War II. In 1939, shortly after the discovery of nuclear fission, the German nuclear energy project, also known as the Uranverein (Uranium Club), had begun. Heisenberg was one of the principal scientists leading research and development in the project.
This program started in April 1939, just months after the discovery of nuclear fission of Uranium in January 1939, but ended only months later, due to the German invasion of Poland, where many notable physicists were drafted into the army. However, the second effort began under the administrative auspices of the German army on the day World War II began (1 September 1939).
From 15 to 22 September 1941, Heisenberg traveled to German-occupied Copenhagen with Carl Friedrich Freiherr von Weizsacker to lecture and discuss nuclear research and theoretical physics with his friend Niels Bohr. These meetings, revealed that Heisenberg and Weizsacker were reluctant to discuss the technical details of the German nuclear program outside of German soil, to do so would have been illegal. The meeting, which was said to have been dominated by Weizacker, was said to have been steered towars the discussion of fundamental nuclear physics, especially the process of the chain reaction in Uranium 238 rather than Uranium 235. This was said to a theoretical discussion, rather than an "intelligence gathering" mission on the development of weapons.
It can be agreed that Heisenberg and Bohr were on good terms after these meetings and after the war itself was over. However, Bohr was genuinely shocked by Heisenberg's implications that Germany was in a secret race to develop a chain reaction in Uranium, considering the large technical effort that Heisenberg had said it required.
Even today there is much controversy whether or not it was Weizsacker or Heiseneberg who was responsible for convincing the Reich to begin a nuclear program and if Heisenberg was reluctant to build a nuclear weapon because he thought it would take too much time and resources or that his own work showed that a chain reaction would not yield a powerful blast. Documents relating to the Bohr-Heisenberg-Weizsacker meetings were released in 2002 by the Niels Bohr Archive and by the Heisenberg family.
On 26 February 1942, Heisenberg presented a lecture to Reich officials on energy acquisition from nuclear fission, after the Army withdrew most of its funding. The Uranium Club was transferred to the Reich Research Council (RFR) in July 1942. On 4 June 1942, Heisenberg was summoned to report to Albert Speer, Germany's Minister of Armaments, on the prospects for converting the Uranium Club's research toward developing nuclear weapons. During the meeting, Heisenberg told Speer that a bomb could not be built before 1945, and would require significant monetary and manpower resources.
The program eventually expanded into three main efforts: the Uranmaschine (nuclear reactor), uranium and heavy water production, and uranium isotope separation. Eventually an experimental nuclear pile called "Atomkellar" (literally "Atom cellar") was developed at the small town of Haigerloch, 40 miles from the university town of Tübingen, from late 1944 until April 1945 as the last large-scale test site for nuclear fission.
The experimental reactor "Atomkellar" after the allied forces defeated Germany
During this time, a research group of the Kaiser Wilhelm Institute for Physics in Berlin, including Werner Heisenberg and Carl Friedrich von Weizsäcker were testing the development of a nuclear reactor with uranium and heavy water as moderator.
When Germany was invaded by the allied forces, Heisenberg had been captured and arrested by Colonel Pash at Heisenberg's retreat in Urfeld, on 3 May 1945, in what was a true alpine-type operation in territory still under control by German forces. He was taken to Heidelberg, where, on the 7th of May, Germany surrendered. Heisenberg did not see his family again for eight months. Heisenberg was moved across France and Belgium and flown to England on 3 July 1945.
Heisenberg was one of ten German scientists were held at Farm Hall in England. The facility had been a safe house of the British foreign intelligence MI6. During their detention, their conversations were recorded. Conversations thought to be of intelligence value were transcribed and translated into English. The transcripts were released in 1992. Bernstein has published an annotated version of the transcripts in his book Hitler's Uranium Club: The Secret Recordings at Farm Hall, along with an introduction to put them in perspective. A complete, unedited publication of the British version of the reports appeared as Operation Epsilon: The Farm Hall Transcripts, which was published in 1993 by the Institute of Physics in Bristol and by the University of California Press in the US
After the war was over, Heisenberg was free to go back to Germany as government, society and infrastructure was rebuilt. Before the war he was the director of the Kaiser Wilhelm Institute for Physical Chemistry at Göttingen (1941-1945) and went back there in 1946 after it was renamed the Max Planck Institute of Physics. He stayed there until 1958, after which he became the professor of Physics at the University of Munich where he remained until his death in 1976.
 Heisenberg, like Bohr, also had meetings with Einstein, weeks before he passed away, and how even then Einstein disliked the idea of quantum mechanics for its inherent uncertainty, although he accepted that quantum mechanics had been rigorously verified through hundreds of experiments. Niels Bohr, who worked closely with Heisenberg was the one who heard Einstein say "God does not play dice", to which Bohr replie "Stop telling God what to do!".
Heisenberg, like Bohr, also had meetings with Einstein, weeks before he passed away, and how even then Einstein disliked the idea of quantum mechanics for its inherent uncertainty, although he accepted that quantum mechanics had been rigorously verified through hundreds of experiments. Niels Bohr, who worked closely with Heisenberg was the one who heard Einstein say "God does not play dice", to which Bohr replie "Stop telling God what to do!". In essence, Einstein's view of quantum mechanics was not of hatred, merely discomfort that the subatomic world is not fully determinable and filled with uncertainty. Einstein did not trust Heisenberg's Uncertainty Principle as a way to describe the universe. However, Heisenberg did think extremely highly of Einstein's theory of special relativity, despite it being nearly outlawed by the Nazi's. Conversely, if "Jewish physics" had actually been banned in Nazi Germany, it would have been extremely bad for Heisenberg and colleagues working on Uranium fission. Therefore, under great personal risk, Heisenberg was willing to put his own safety on hold to discover the truth and would not have cared, in the same way Einstein would have, if the laws of physics seem displeasing.
By knowing that the uncertainty is inherent, Heisenberg was certain that the behaviour of quantum particles is based on probability and statistics and that these give us the power to go beyond classical thinking, something which continues to drive physics into the great unknown.
NOTES:
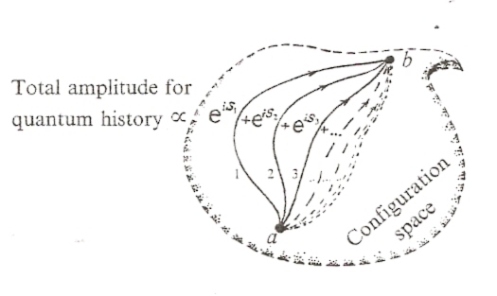
In quantum theory, the action, S, is utilized to calculate a quantum amplitude. The basic idea is the sum over all possible paths. In any kind of phase space of dynamical variables we want to consider, the configuration state of any possible system is represented in terms of those dynamical variables. The quantum amplitude that describes the dynamical evolution of any system from one configuration state to another configuration state is defined by a sum over all possible paths that connect those initial and final configuration states:
Each path is weighted with a probability factor ψ that depends on the action S for that path as
ψ=exp(2πiS/h)
The total quantum amplitude is a sum over all possible paths, and is understood in the sense of quantum probability. The probability factor ψ for any given path gives the quantum probability that the system will actually follow that particular path. The total quantum amplitude represents a quantum state of potentiality. In actuality, the system actually follows a particular path. The likelihood that the system will actually follow a particular path is determined by the probability factor ψ for that particular path, which depends on the action for that path.
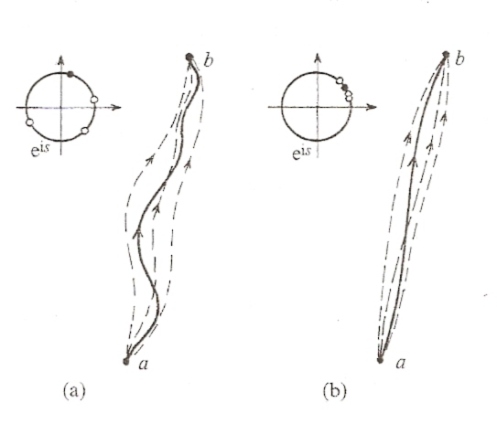
The most likely path in the sense of quantum probability is the path of least action. In some sense, the path of least action is like the path of maximal phase reinforcement in an interference pattern. When the waves are in phase with each other they tend to add together, and when the waves are out of phase with each other they tend to cancel out:
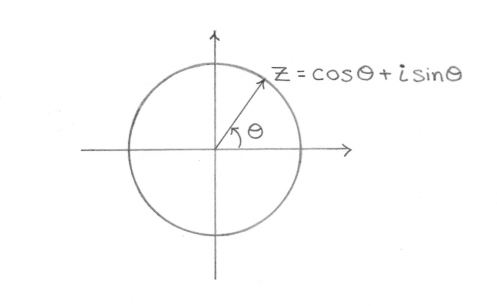
This phenomenon occurs in the total quantum amplitude since each probability factor ψ behaves like a wave. This phenomenon is inherently related to the nature of the complex plane, where the complex number z=exp(iθ)=cos(θ)+isin(θ) acts like a unit vector with a phase angle θ in the complex plane:
where e is the base of the natural logarithm, i is the imaginary unit, and cos and sin are the trigonometric functions cosine and sine respectively, with the argument x given in radians.
The path of least action arises since the unit vector, z, tend to cancel out when the phase angles are out of phase with each other, and the vectors tend to add together when the phase angles are in phase with each other:
The path of least action is the most likely path in the sense of quantum probability. In actuality the system actually follows some particular path. In the case of a single photon in the double slit experiment, the photon acts like a particle that follows some actual path. The most likely path is the path of least action, but when we superimpose the behavior of many different individual photons we discover the interference pattern:
In the sense of quantum theory, every event is a decision point where the quantum state of potentiality branches into all possible paths.
Remember that since each particle path is weighted by the probability factor ψ, which is scaled by Planck's constant h, for amplitudes summed up over configuration spaces where Planck's constant becomes negligible the deviation from the least action (dS=0, i.e. a straight line) will become equally negligible. Hence we do not see quantum behavior in macroscopic configuration spaces, which is in everyday, ordinary, incoherent matter.