Using Unmanned Aerial Vehicles (UAVs or "Drones") we can do scanning in the visual spectrum of large areas in the surrounding environment without the high comparative cost of using manned vehicles such as helicopters or planes or expensive space-based monitoring infrastructure. UAVs allow for fast, local and energy efficient surveillance for environmental monitoring.
In the case of ecosystem analysis, it is key to have active monitoring of plant health and distribution in order to gauge the health of an ecosystem and the support potential for the various species that depend on healthy, diverse and broad growth of vegetation.
Applications of UAVs in environmental protection, forestry and plant agriculture agriculture, forestry and environmental protection include:
- Local Ecosystem Monitoring
- High Frequency Vegetation Growth Analysis
- Monitoring of Agricultural Impact on Environment
- Empiracle Measurement of Unidentified Vegetation Die-off (UVD)
- Monitoring of Agricultural Impact on Environment
- Monitoring Health of Agricultural Plant Crops
- Detection of Water/Soil Stress on Plants
- Early Detection of Disease and Pest impact on Plants
- Monitoring Pollution and Spill Impacts on Plants
UAV Drones can provide a fast, cheap, efficient (both in energy and in time) and yet very effective way to perform environmental diagnostics and information retrieval without depending on more complex infrastructure such as satellite and manned aircraft.
UAVs can be used in crop monitoring and within what is called "precision agriculture", which works in order to optimize plantation management and assess more accurately the optimum density planting, in addition to making decisions regarding the use of fertilizers, irrigation frequency and other possibilities, such as to predict more accurately the crop production and allowing for sustainable use of the limited resources of water, soil and land available for agriculture.
The UAV can perform scheduled flights to carry out surveys of areas of vegetation (an indicated use, for example, for the of monitoring vulnerable ecosystems), and compare the spectral data taken from sensor cameras with available visual tomography, provided by accurate and updated maps, to detect and deduce root causes of detected instances of stress in the areas of plant growth.
In the case of the visual tomography we can update existing maps with relative ease by simply referencing 2D aerial or satellite survey maps with the measured heights at certain locations in order to predict water flow and effects of soil creeping and leeching. Several map providers and simulation tools exist for modelling water flows in an environment, all of which is a discussion for another time.
However, with the sensor information we need to think a little more abstractly and find key variables which indicate the state of plant health.
Near-Infrared (NIR) and Plant Health
Near-Infrared, NIR is a small portion of the much larger region called infrared (IR), located between the visible and microwave portions of the electromagnetic (EM) spectrum.
NIR makes up the part of IR closest in wavelength to visible light and occupies the wavelengths between about 700 nanometers and 1500 nanometers (0.7 µm – 1.5 µm). NIR is not to be confused with thermal infrared, which is on the extreme other end of the infrared spectrum and measures radiant (emitted) heat.
In most commercially available cameras, most of which based on silicon semiconductor Charged-Coupled Devices (CCD) detectors, absorb visible light from about 390nm until about 1200 nm,
NIR radiation can be blocked by special glass or plastic windows designed to ensure natural colour images are produced without undesired shifts towards the extremes of the red or blue parts of the EM spectrum, the shift depending on the nature of the semiconductor CCD sensor. So-called "hot mirrors" are used to negate the influence of the NIR on camera images which would otherwise induce a reddening effect.
A replacement of the hot mirror by a neutral glass or plastic filter can therefore be used for infrared photography. As a consequence of this a significant increase of commercial camera modularity can be achieved by this special optical set up and users can decide whether they want to generate natural colour (true colour) or near infrared (NIR) images, depending on the external lens filter sets applied. This allows the camera to detect the infrared light necessary for producing NDVI images.
Using multispectral and in particular near-infrared (NIR) surface reflectance cameras, the various monitoring parameters for vegatation can be gained easily, sometimes in a single flight sweep over an area, to generate quality indicators of plant health.
When studying the vegetation reflectance spectrum in detail, The near-infrared spectrum itself allows a relatively high detailed probe of the health of plants at a cellular level.
In photosynthesis, the chloroplasts in plant cells take absorb light energy sing chlorophyll which aborbes photons and creates and electronic channel to fix carbon and water to form glucose, the basis of carbohydrates and hence food for the plant.
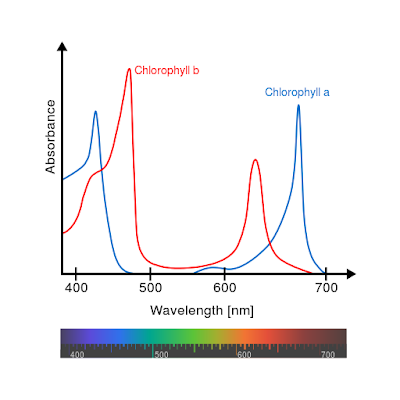
The forms of chlorophyll in plants absorb light at specific frequencies, typically, in the red and blue light. The green portion of light is effectively reflected, this makes the plant was seen in the range of green in the visible spectrum.
This information is also important for developing lighting systems, for indoor agriculture for example, of which light frequencies are the most efficient for growing plants as exploited by so-called "grow lights" for indoor plant growth. This is another topic in precision agriculture which can be explored further.
Unhealthy vegetation, on the other hand, will have less chlorophyll and thus will appear brighter (visibly) since less is absorbed and more is reflected to our eyes. This increase in red reflectance along with the green is what causes a general yellow appearance of unhealthy plants.
Plants reflect strongly in the NIR however not because of chlorophyll but because of a spongy layer of lignin found on the bottom surface of the leaf, but not strongly in the red.
IR reflectance is advantageous to plants, as it is reflects electromagnetic energy that the plant cannot use and moreover would probably damage the plant tissues, especially during high levels of sunshine where the IR radiation would heat the plant tissues and slow down or damage cellular processes.
Plant stress causes an increase in visible light transmission in the green as the chlorophyll decays and the much more obvious effect of an increase in the reflectance of red, this is why the leaves of deciduous trees turn orange and red when they die-off in the autumn.
The near-infrared plateau (NIR, 700 nm - 1100 nm), is a region where biochemical absorptions are limited to the compounds typically found in dry leaves, primarily cellulose, lignin and other structural carbohydrates.
However, and this is critical, NIR reflection in this region is also affected by multiple scattering of photons within the leaf, related to the internal cellular structure, fraction of air spaces in the xylem vessels of the plant, and most important as a general indicator, the air-water interfaces that refract light within leaves. The reflectance and transmittance in the middle-infrared also termed the shortwave-infrared (SWIR, 1100 nm - 2500 nm) is also a region of strong absorption, primarily by water in green leaves. The primary and secondary absorptions of water in leaf reflectance are greatest in spectral bands centered at 1450, 1940, and 2500 nm, with important secondary absorptions at 980 nm, and 1240 nm (Carter, 1991). These are the bands which create the primary NIR reflectance in healthy plants.
The cohesion-adhesion model of water transport in vascular plant tissue describes how hydrogen bonding in water to explain many key components of fluid movement through the plant's xylem and other vessels.
Within a vessel, water molecules hydrogen bond not only to each other, but also to the cellulose chain itself which comprises the wall of plant cells. This creates a capillary tube which allows for capillary action to occur since the vessel is relatively small. This mechanism allows plants to pull water up into their roots. Furthermore,hydrogen bonding can create a long chain of water molecules which can overcome the force of gravity and travel up to the high altitudes of leaves.
Cohesion-adhesion model of water transport in cellulose-based plant vascular tissue
Soil, on the other hand, reflects both NIR and Red . However, when a plant becomes dehydrated or sickly, the spongy layer collapses and the lack of water itself and its interfacing with the plant all contributes to ceasing to reflect as much NIR light. Thus, a combination (approximated as linear) of the NIR reflectivity and red reflectivity should provide excellent contrast between plants and soil and between healthy plants and sick plants.
The Normalized Difference Vegetation Index (NDVI) is a simple graphical indicator that can be used to analyze remote sensing measurements, such as in aerial and space based surveys, and assess whether the target being observed contains live green vegetation or not.
It turns out which combination is not particularly important, but the NDVI index of (NIR-red)/(NIR+red) does happen to be particularly effective at normalizing for different irradiation conditions.
Hence, plants in a given area that are adequately hydrated show high absorbance of NIR light in this absorbance band (and low reflectance), whereas those subject to drying shows greater reflectance in this band.
Specifically, NDVI was developed by a NASA scientist Dr. Compton Tucker in a 1977 paper
entitled, “Red and Photograghic Infrared Linear Combinations for Monitoring Vegetation.”
Tucker examined 18 different combinations of NIR (Landsat MSS 7 800-1100 nm), red (Landsat MSS 5 600-700 nm), and green (Landsat MSS 4 500-600 nm) and compared these results with the density of both wet and dry biomass to in an attempt determine which combination correlated best.
His findings were that
- NIR/red,
- SQRT(NIR/red),
- NIR-red,
- (NIR-red)/(NIR+red),
- SQRT((NIR-red)/(NIR+red)+0.5)
were all very similar indicators for estimating the density of photosynthetically active biomass.
Using this a threshold level for near IR reflectance from healthy plants can be deduced, allows for a way to label plant health remotely.
Using a near-infrared spectral camera, a drone can easily monitor vegetation for signs of sickness and determine the health of both agricultural crops and the plants at the base of a foodchain in ecosystems to monitor an environment which is in a constant state of change. This is of utmost importance in parts of the world which are suffering from environmental destruction, both natural and increasingly induced by humans. The surveillance of vegetation in endangered areas is of very high importance and new methods need to be introduced to ensure the survival of the most vulnerable biomes on earth, namely the tropical, temperate and boreal forests.
A common NIR Filter is the "Congo Blue" gel filter which passes NIR, which is detected as red by a CCD sensor, and does not allow green to pass.
"Congo Blue" filter for one window of the multi-spectral camera
Such split screen lenses can be included on UAV cameras as well as on high-definition portable camera technologies, i.e. smartphones, which can be light enough (and which are now so ubiquitous) that they can be used as diagnostic instruments for ecosystem monitoring.
Carbon-fiber drone fitted with split-screen camera
Using these techniques, a portable UAV with a camera fitted with a dual near-infrared + true-color lens fitting to fly over such an environment that lies close to a town area to monitor vegetation health.

The experimental data can then be fed back via Wifi feed to a smartphone or a computer, from which the data can be analysed using computer software to retrieve vegetation information from surveillance drone by calculating the vegetation index and overlaying the indices pixel by pixel on the composite image.
Developing this further we could also make a live NIR-camera feed application for use on portable computer, i.e. tablets and smartphone, technology which can allow for fast and easy remote diagnostics of plant health.
The stereoscopic split-screen lens mount is universally adaptable for use in smartphones, so for local use, or for active viewing on a height say, the diagnostic can be done entirely on portable computer infrastructure itself. It is hoped that such apps can become more widespread and applicable for simple to install multi-spectral camera attachments.
All of this could save energy and finance for performing the relatively simple step of diagnosis of plant health and help spur-on the the real work of finding solutions to remedy the problem of plant disease, dehydration and malnutrition in an environment.
Notes:
Since both N and O are strongly electronegative, the hydrogen atoms bonded to nitrogen in one polypeptide backbone can hydrogen bond to the oxygen atoms in another chain and visa-versa. Though they are relatively weak,these bonds offer great stability to secondary protein structure because they repeat a great number of times.